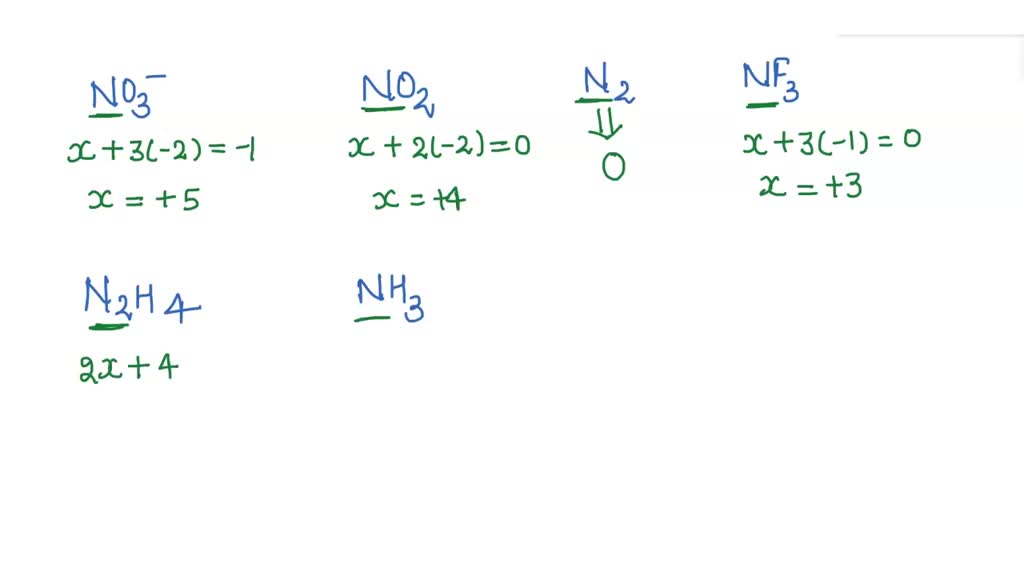Nitrogen Oxide Oxidation Number . enter the formula of a chemical compound to find the oxidation number of each element. Often when assigning oxidation numbers, it is. Since oxygen is slightly more electronegative than. — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. It exhibits different oxidation states in its oxides, ranging from +1 to +5. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). — n in n o has a formal oxidation number of +i i. nitrogen reacts with oxygen to form a number of nitrogen oxides. A net ionic charge can be specified at the. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than those in, the lower oxidation state.
from www.numerade.com
Since oxygen is slightly more electronegative than. Often when assigning oxidation numbers, it is. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). — n in n o has a formal oxidation number of +i i. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than those in, the lower oxidation state. A net ionic charge can be specified at the. It exhibits different oxidation states in its oxides, ranging from +1 to +5. — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. enter the formula of a chemical compound to find the oxidation number of each element. nitrogen reacts with oxygen to form a number of nitrogen oxides.
SOLVED Oxidation numbers of nitrogen For the following compounds, rank
Nitrogen Oxide Oxidation Number nitrogen reacts with oxygen to form a number of nitrogen oxides. A net ionic charge can be specified at the. Often when assigning oxidation numbers, it is. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than those in, the lower oxidation state. — n in n o has a formal oxidation number of +i i. nitrogen reacts with oxygen to form a number of nitrogen oxides. It exhibits different oxidation states in its oxides, ranging from +1 to +5. — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Since oxygen is slightly more electronegative than. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). enter the formula of a chemical compound to find the oxidation number of each element.
From hadassah-blogbonilla.blogspot.com
Determine the Oxidation State of Nitrogen in No2. Nitrogen Oxide Oxidation Number A net ionic charge can be specified at the. — n in n o has a formal oxidation number of +i i. enter the formula of a chemical compound to find the oxidation number of each element. Since oxygen is slightly more electronegative than. nitrogen reacts with oxygen to form a number of nitrogen oxides. It exhibits. Nitrogen Oxide Oxidation Number.
From www.doubtnut.com
[Odia] What is the oxidation number of nitogen in nitrous oxide. Nitrogen Oxide Oxidation Number Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than those in, the lower oxidation state. Often when assigning oxidation numbers, it is. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). nitrogen reacts with oxygen to form a number of nitrogen oxides. enter the formula of a chemical compound to. Nitrogen Oxide Oxidation Number.
From www.scribd.com
Oxidation Numbers of Nitrogen Redox Nitrogen Nitrogen Oxide Oxidation Number It exhibits different oxidation states in its oxides, ranging from +1 to +5. Often when assigning oxidation numbers, it is. nitrogen reacts with oxygen to form a number of nitrogen oxides. — n in n o has a formal oxidation number of +i i. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than. Nitrogen Oxide Oxidation Number.
From www.chemistrylearner.com
Oxidation Number (State) Definition, Rules, How to Find, and Examples Nitrogen Oxide Oxidation Number — n in n o has a formal oxidation number of +i i. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). nitrogen reacts with oxygen to form a number of nitrogen oxides. It exhibits different oxidation states in its oxides, ranging from +1 to +5. enter the formula of a chemical compound to. Nitrogen Oxide Oxidation Number.
From classnotes.org.in
Oxides of Nitrogen Chemistry, Class 12, The pBlock Elements Nitrogen Oxide Oxidation Number enter the formula of a chemical compound to find the oxidation number of each element. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). — n in n o has a formal oxidation number of +i i. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than those in, the lower. Nitrogen Oxide Oxidation Number.
From www.numerade.com
SOLVEDThe following equations show redox reactions that are sometimes Nitrogen Oxide Oxidation Number Often when assigning oxidation numbers, it is. — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). — n in n o has a formal oxidation number of +i i. enter the formula of. Nitrogen Oxide Oxidation Number.
From www.researchgate.net
Processes in the microbial nitrogen cycle. Oxidation states of each Nitrogen Oxide Oxidation Number nitrogen reacts with oxygen to form a number of nitrogen oxides. Often when assigning oxidation numbers, it is. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. A net ionic charge can be specified. Nitrogen Oxide Oxidation Number.
From www.numerade.com
SOLVED Oxidation numbers of nitrogen For the following compounds, rank Nitrogen Oxide Oxidation Number the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). A net ionic charge can be specified at the. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than those in, the lower oxidation state. — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate. Nitrogen Oxide Oxidation Number.
From www.numerade.com
Nitrogen can have several different oxidation numbers ranging in value Nitrogen Oxide Oxidation Number A net ionic charge can be specified at the. — n in n o has a formal oxidation number of +i i. Since oxygen is slightly more electronegative than. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than those in, the lower oxidation state. It exhibits different oxidation states in its oxides, ranging from. Nitrogen Oxide Oxidation Number.
From www.youtube.com
How to Balance N2 + O2 = NO2 (Nitrogen gas + Oxygen gas) YouTube Nitrogen Oxide Oxidation Number A net ionic charge can be specified at the. Often when assigning oxidation numbers, it is. nitrogen reacts with oxygen to form a number of nitrogen oxides. Since oxygen is slightly more electronegative than. — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. — n. Nitrogen Oxide Oxidation Number.
From www.numerade.com
SOLVEDWrite chemical formulas for oxides of nitrogen with the Nitrogen Oxide Oxidation Number nitrogen reacts with oxygen to form a number of nitrogen oxides. — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. A net ionic charge can be specified at the. Since oxygen is slightly more electronegative than. — n in n o has a formal oxidation. Nitrogen Oxide Oxidation Number.
From www.nagwa.com
Question Video Identifying the Oxidation Number of Nitrogen in Nitric Nitrogen Oxide Oxidation Number A net ionic charge can be specified at the. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than those in, the lower oxidation state. nitrogen reacts with oxygen to form a number of nitrogen oxides. — n in n o has a formal oxidation number of +i i. the oxidation number of. Nitrogen Oxide Oxidation Number.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Nitrogen Oxide Oxidation Number nitrogen reacts with oxygen to form a number of nitrogen oxides. It exhibits different oxidation states in its oxides, ranging from +1 to +5. A net ionic charge can be specified at the. enter the formula of a chemical compound to find the oxidation number of each element. Since oxygen is slightly more electronegative than. — n. Nitrogen Oxide Oxidation Number.
From www.youtube.com
How to find Oxidation Numbers for Nitrogen (N) YouTube Nitrogen Oxide Oxidation Number enter the formula of a chemical compound to find the oxidation number of each element. Often when assigning oxidation numbers, it is. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). — n in n o has a formal oxidation number of +i i. nitrogen reacts with oxygen to form a number of nitrogen. Nitrogen Oxide Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for N in N2O ( Nitrous oxide) YouTube Nitrogen Oxide Oxidation Number the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Since oxygen is slightly more electronegative than. nitrogen reacts with oxygen to form a number of nitrogen oxides. Oxides of nitrogen having nitrogen in the. Nitrogen Oxide Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for N in NO (Nitrogen monoxide) YouTube Nitrogen Oxide Oxidation Number A net ionic charge can be specified at the. Often when assigning oxidation numbers, it is. nitrogen reacts with oxygen to form a number of nitrogen oxides. enter the formula of a chemical compound to find the oxidation number of each element. — n in n o has a formal oxidation number of +i i. the. Nitrogen Oxide Oxidation Number.
From pubs.rsc.org
Converting between the oxides of nitrogen using metalligand Nitrogen Oxide Oxidation Number enter the formula of a chemical compound to find the oxidation number of each element. Since oxygen is slightly more electronegative than. the oxidation number of the nitrogen atom in \(\ce{hno_3}\) is \(+5\). — n in n o has a formal oxidation number of +i i. Often when assigning oxidation numbers, it is. nitrogen reacts with. Nitrogen Oxide Oxidation Number.
From www.youtube.com
How to calculate the oxidation number of Nitrogen in N2O(Nitrous oxide Nitrogen Oxide Oxidation Number — n in n o has a formal oxidation number of +i i. — the oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Since oxygen is slightly more electronegative than. A net ionic charge can be specified at the. It exhibits different oxidation states in its oxides,. Nitrogen Oxide Oxidation Number.